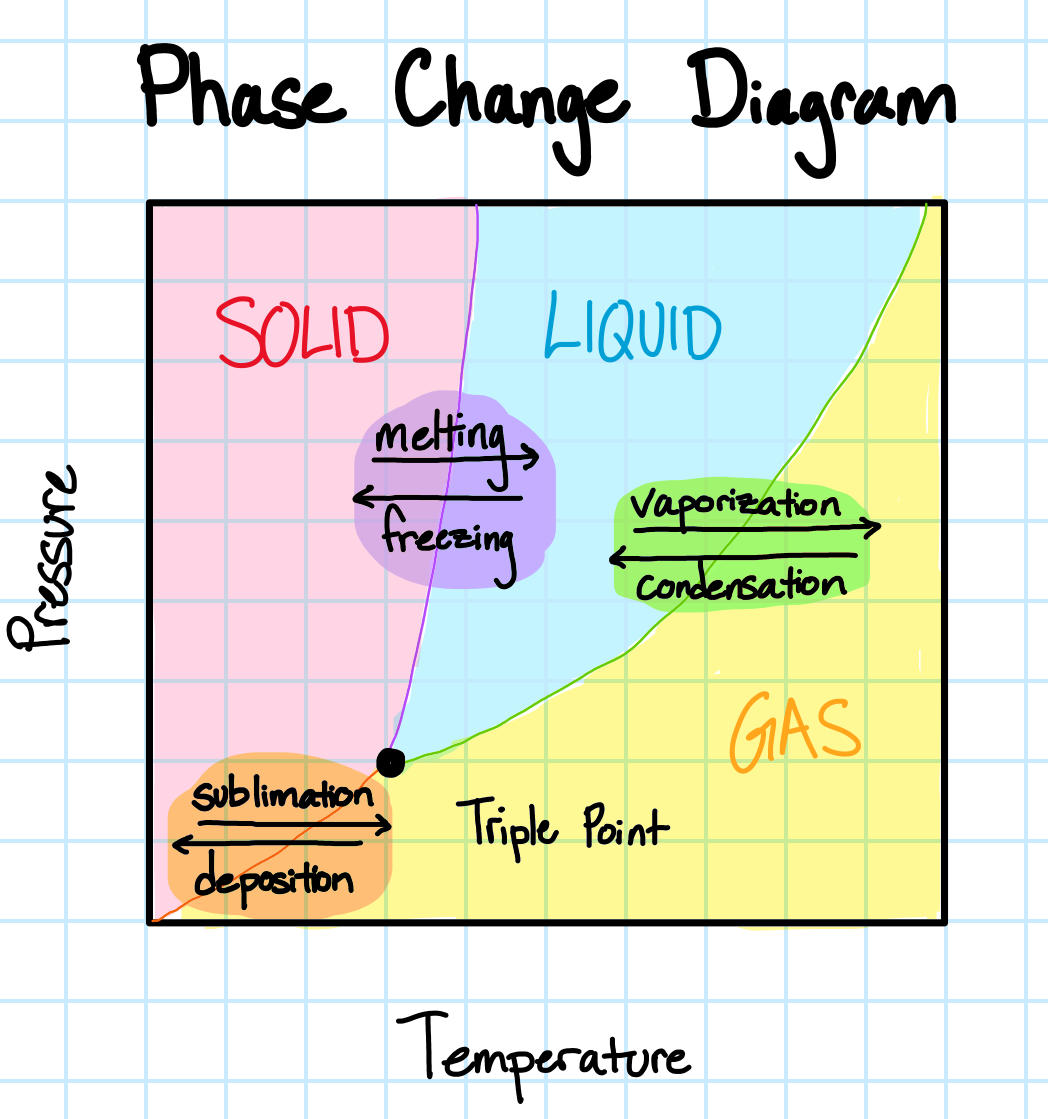
Change of Phase
Phase changes complicate heat transfer, as the process is energy intensive and causes changes in the properties of the material. Affected properties include volume, density, thermal conductivity, specific heat, dielectric properties (i.e. the interaction of a material with electromagnetic waves), and mechanical properties (i.e. elastic modulus)
For example, consider the cooling of water from vapor to liquid to ice:
- Density increases. Solids have the highest density, since atoms are tightly packed together.
- Volume decreases. As the density of a material increases, volume decreases.
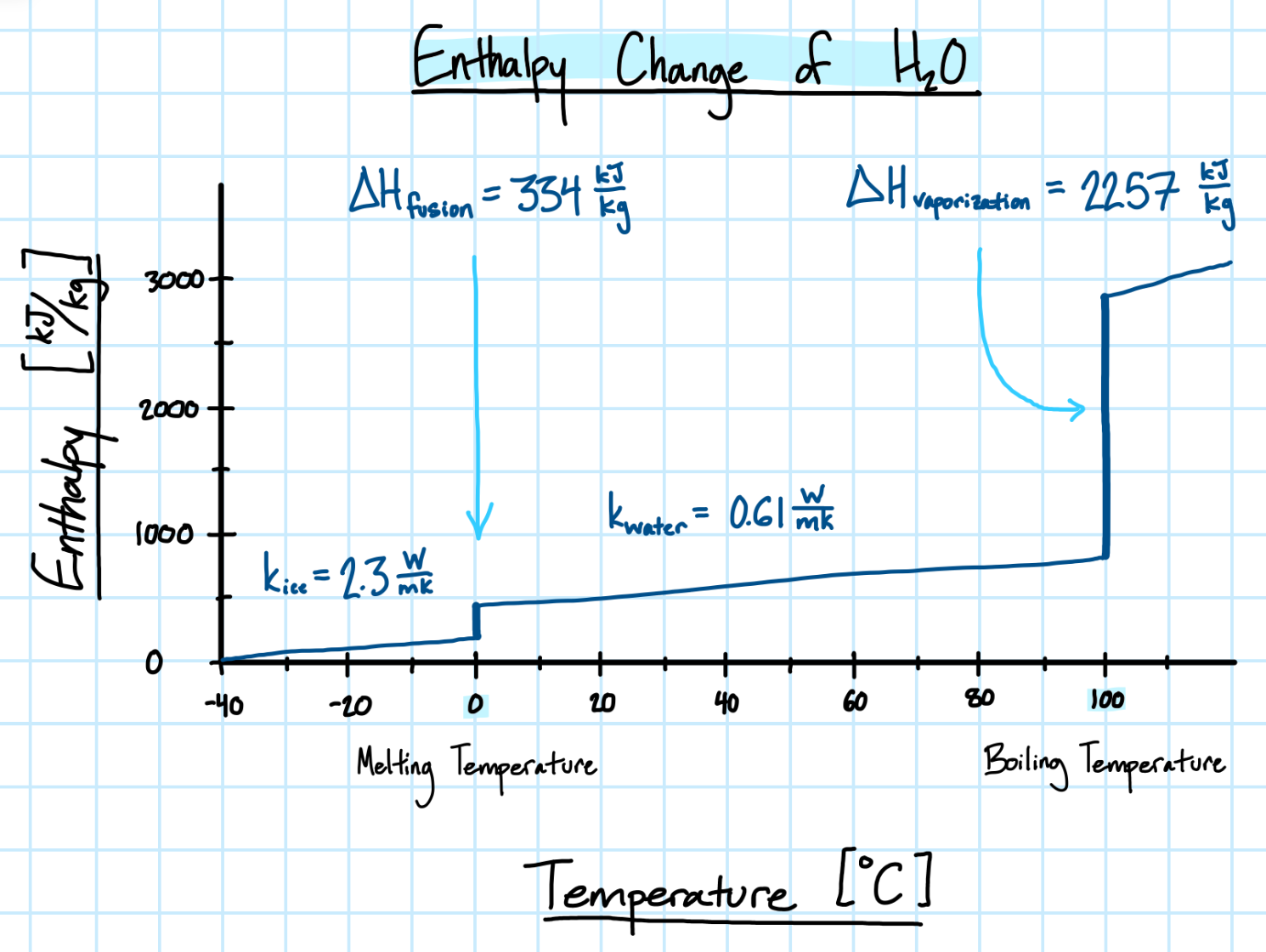
- Thermal conductivity increases. The thermal conductivity of ice is nearly three times greater than water.
- Specific heat slightly decreases. Generally, \( C_{p, gas} < C_{p, solid} \leq C_{p, liquid} \) Intuitively, liquids buffer temperature best.
- Dielectric properties decrease. For example, frozen food takes much longer to microwave, due to poor microwave absorption.
- Elastic modulus decreases. Typically, materials become more brittle at colder temperatures.
As a material changes phase, so does the energy of the system. The latent heat of fusion (\( \Delta H_{fus} \) ) describes the enthalpy difference due to a change of phase from solid to liquid, which occurs at melting temperature. Conversely, the latent heat of vaporization (\( \Delta H_{vap} \) ) describes the enthalpy difference due to a change of phase from liquid to vapor, which occurs at boiling temperature.