What is Transport?
Transport refers to how energy, mass, and momentum move through systems, and it is a core idea in biology, the environment, and engineering. These processes are essential for the function of living systems, operate across many scales, and are critical to the design and function of bioengineered technologies.
To fully understand transport, it is important to combine biological context and mathematical tools to analyze living systems such as plants, animals, and the human body, as well as natural systems like air, water, and soil. Unlike purely mechanical or chemical systems, biological systems involve additional complexities, including metabolism, heat production, and oxygen use, which affect how transport occurs. Transport can be split into biomedical, plant, bioprocessing, and bioenvironmental contexts.
Transport in a Biomedical Context
Transport in biomedical context occurs at the sub-cellular, cellular, transcellular, tissue/organ, whole body, populations levels. Some examples include thermoregulation, metabolism, bioheat transfer, blood as oxygen carrier, membrane transport, and liquid diffusion in tissue.
Understanding transport at these levels can help with clinical and bioengineering applications such as cryosurgery, laser ablation of tumors, drug dosing, dialysis machines, artificial organs, gene delivery, and cryopreservation.
Modes of Heat & Mass Transfer Overview
Transport phenomena describe how energy, mass, and momentum move through systems, generally biological and engineered systems. For our purposes, we will be focusing primarily on heat transport (the transfer of thermal energy) and mass transfer (the movement of chemical species).
An important principle to understand is that transport occurs when a driving force exists. For heat transfer, the driving force is a temperature gradient. For mass transfer, the driving force is a concentration gradient. These transport processes reduce gradients over time, moving the system toward equilibrium.
In the next few sections, these primary mechanisms of heat and mass transport will be introduced before a more detailed exploration later.Conduction
Conduction is a mode of heat transfer that occurs through direct molecular motion within a material, without any bulk motion of the material itself. When a temperature gradient exists within a solid, thermal energy is transferred from regions of high temperature to regions of lower temperature as molecules randomly interact.
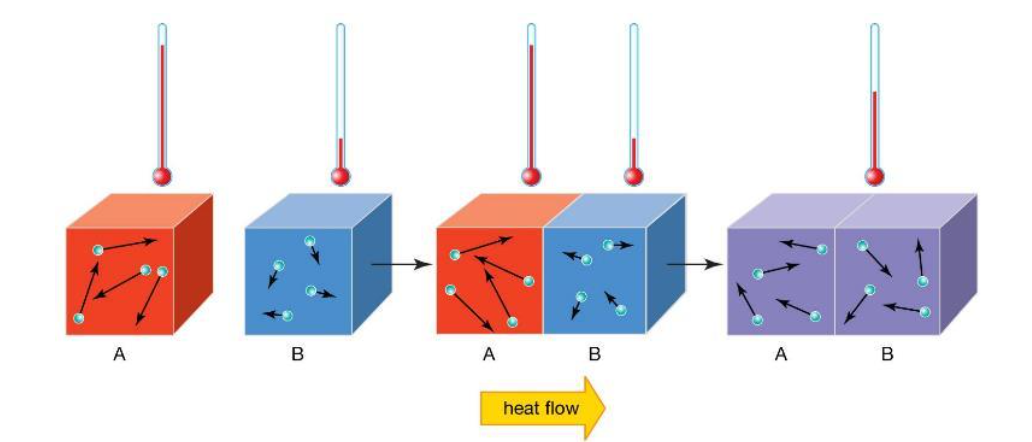
At the molecular level, conduction functions by random molecular motion and collisions. As you can see in Figure 1, when two solids at different temperatures are placed in contact, thermal energy is transferred from the hotter material to the cooler object until thermal equilibrium is reached.
Convection
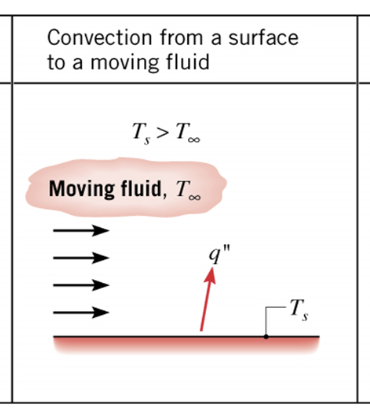
Convection is a mode of heat transfer that occurs due to the bulk motion of a fluid, such as air, water, or blood. A fluid is any substance that can flow, including both liquids and gases. In Figure 2, a solid surface is hotter than the fluid flowing past it, so heat moves from the surface into the fluid. As the fluid flows, it carries heat away from the surface.
Radiation
Radiation is a mode of heat transfer that occurs due to the emission and absorption of electromagnetic waves and does not require direct contact or fluid motion. A familiar example of radiative heat transfer might be feeling warmth from the sun. In biological systems, radiation contributes to heat exchange between the body and the environment, as the body continuously emits infrared radiation into its surroundings.
Diffusion
Diffusion is a mode of mass transfer driven by the random motion of molecules. When a concentration gradient exists, molecules move randomly, but on average, there will be a net movement of species from regions of higher concentration to lower concentration. Diffusion doesn’t require bulk fluid motion.
Example: Drop of colored dye spreading through still water over time. Even though the water itself is not moving, the dye molecules will disperse until achieving a uniform concentration.
Similarities between Heat & Mass Transfer
Heat and mass transfer are mathematically and conceptually analogous transport processes governed by gradients, material properties, conservation laws, and shared modes of diffusion and convection.
Both transport processes describe the movement of an extensive quantity driven by gradients and are governed by conservation laws. Gradients drive transport of heat and mass, generating flux. This flux is resisted by the heat conductance of the medium, or by the molecular diffusivity of the chemical species. For example, Ohm's Law governs the flow of electrons (ie. current), driven by voltage and hindered by resistance.
| Heat Transfer | Mass Transfer | |
|---|---|---|
| What is Moving | Thermal energy | Chemical species |
| Conserved Quantity | Energy | Mass |
| Driving Force | Temperature gradient | Concentration gradient |
| Resistive Element | Thermal conductivity (k) | Molecular diffusivity (D) |
Table 1. A comparison of variables considered in heat and mass transfer.
Heat and mass transfer differ in their modes of transport. While heat transfer has three modes of transport (conduction, convection, and radiation) as explored above, mass transfer has diffusion, dispersion, and pressure-driven transport.
Heads up!
Modes of heat and mass transfer builds on this content later in the course.
Learn more about this topic on the Heat Transfer Mechanisms and Mass Transfer Mechanisms pages.