Modes of Heat & Mass Transfer
Transport phenomena describe how energy, mass, charge, and momentum move through systems, whether biological or engineered systems. These transport processes reduce gradients over time, moving the system toward equilibrium. An important principle to understand is that transport occurs when a driving force exists, which is proportional to flow. At the same time, a resistance to flow will impede its motion. The flow relationship is described in an Ohm's Law type of formulation. For example, the fluid flow through a blood vessel, \( q = \frac{ΔP}{R} \), is proportional to the pressure difference across the vessel divided by the resistance throughout the vessel.
These Reference Pages will focus primarily on heat transport (the transfer of thermal energy) and mass transfer (the movement of chemical species). In the next few sections, primary mechanisms of their transport are introduced.
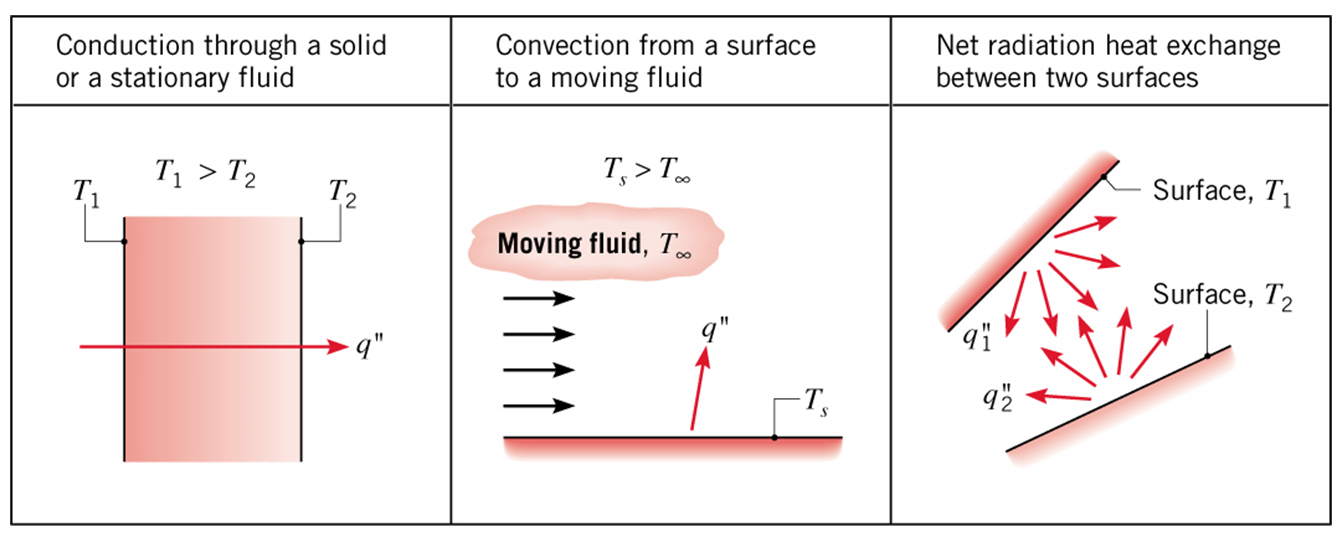
Conduction
Conduction occurs through direct molecular motion within a material, without any bulk motion of the material itself. When a temperature gradient exists within a solid, thermal energy is transferred from regions of high temperature to regions of lower temperature as molecules randomly collide. When two solids at different temperatures are placed in contact, thermal energy is transferred from the hotter material to the cooler object until thermal equilibrium is reached (i.e., the objects are at the same temperature).
Convection
Convection occurs due to the bulk motion of a fluid. A fluid is any substance that can flow, including both liquids and gases such as air, water, or blood. As it flows, it carries heat away from the surface.
Radiation
Radiation occurs due to the emission and absorption of electromagnetic waves. It does not require direct contact or fluid motion. A familiar example of radiative heat transfer is feeling warmth from the sun. In biological systems, radiation contributes to bidirectional heat exchange between the system and its surroundings, as the body can continuously emit and absorb infrared radiation.
Diffusion
Diffusion is a mode of mass transfer driven by the random motion of molecules. When a concentration gradient exists, molecules move randomly, but on average, there will be a net movement of species from regions of higher concentration to lower concentration. Diffusion doesn’t require bulk fluid motion.